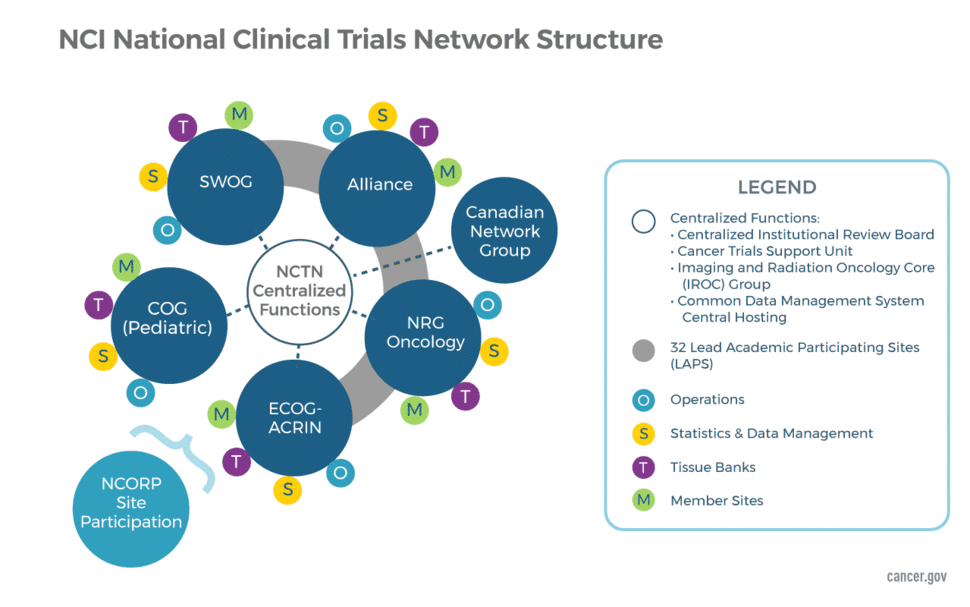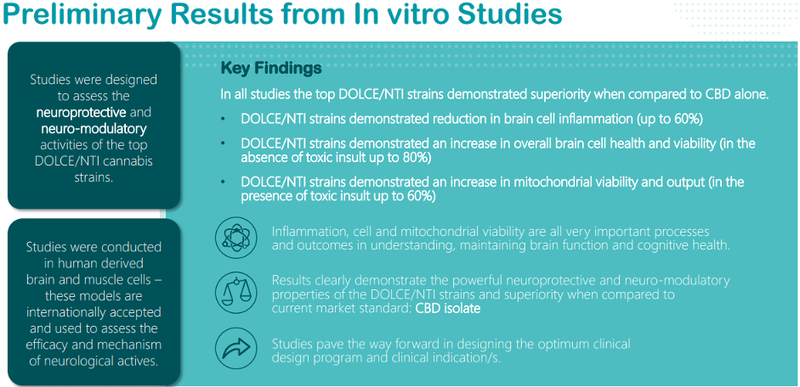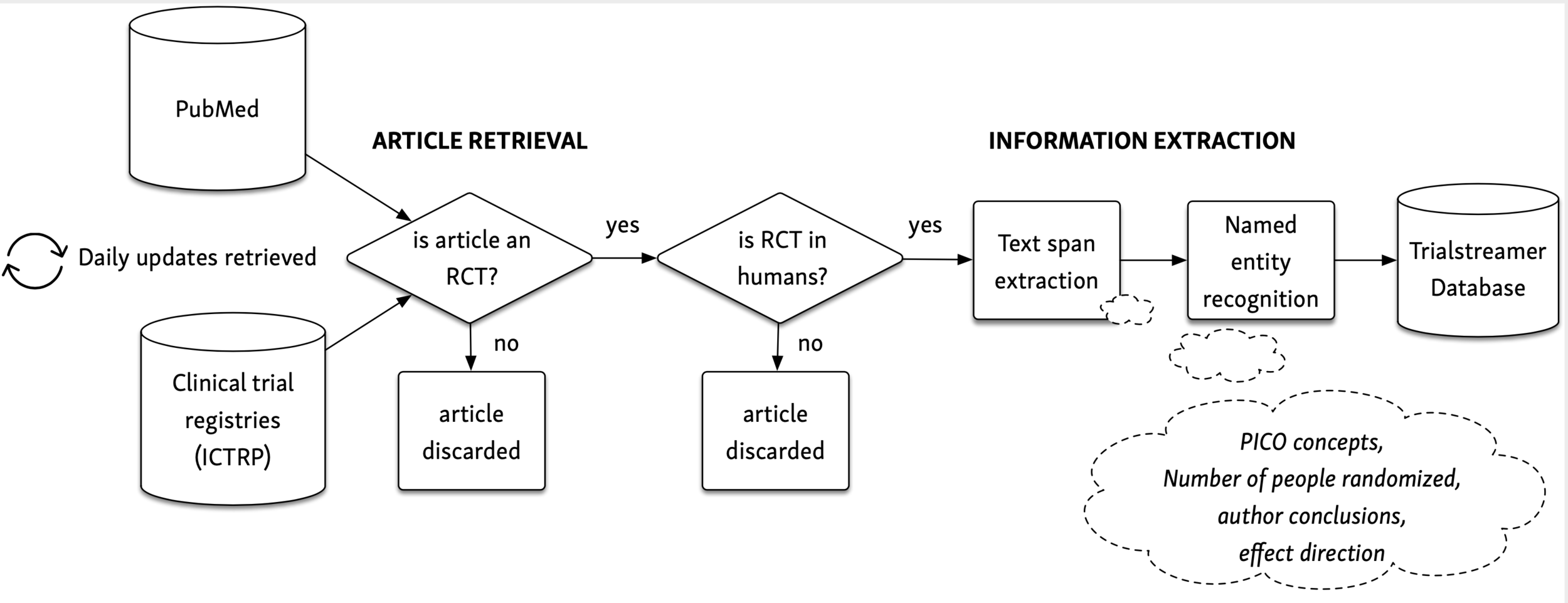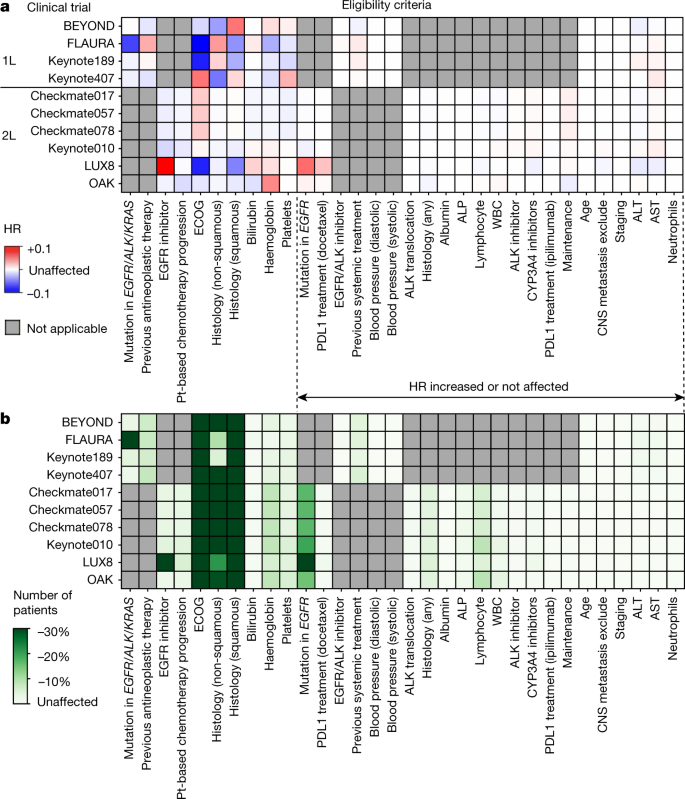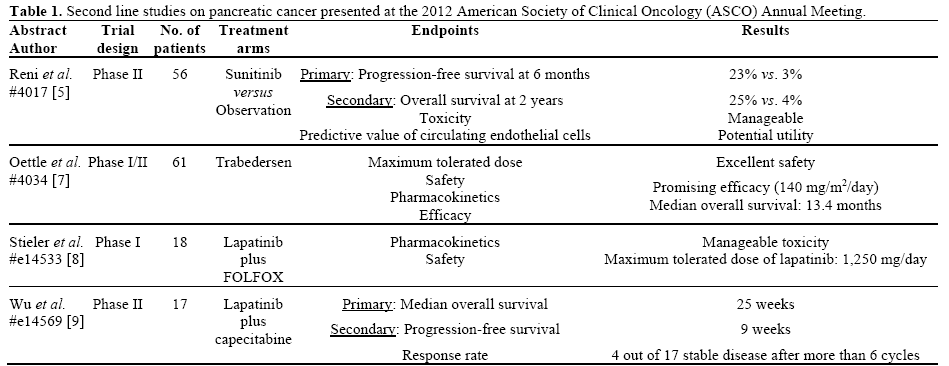
Clinical Studies in the Second Line Setting of Advanced Pancreatic Cancer: Are We Making Any Progress? | Insight Medical Publishing

Generalizability of findings from randomized controlled trials is limited in the leading general medical journals - Journal of Clinical Epidemiology

Barriers and enablers to locally-led clinical trial conduct in low and middle income countries: strategies for developing locally sustainable health research capacity | Semantic Scholar

PDF) Clinical Trial Evidence Supporting US Food and Drug Administration Approval of Novel Cancer Therapies Between 2000 and 2016

Accessibility of trial reports for drugs stalling in development: a systematic assessment of registered trials – topic of research paper in Clinical medicine. Download scholarly article PDF and read for free on
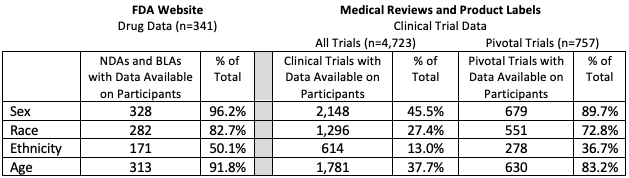
Demographic Disparities in Patient Samples for Drugs and Biologics Approved by FDA Between 2007-2017
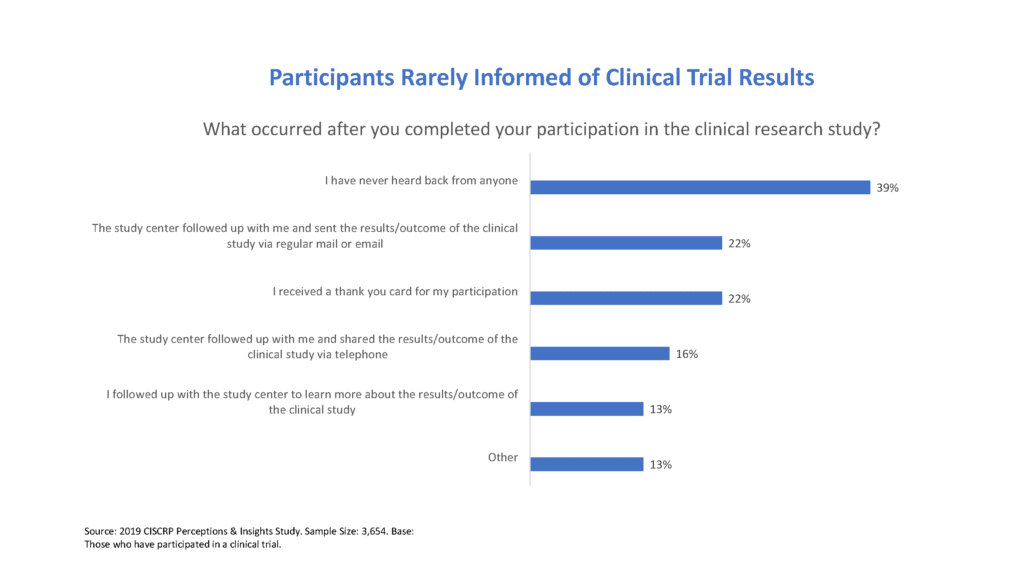
CISCRP's 2019 Perceptions and Insights Study - Center for Information & Study on Clinical Research Participation